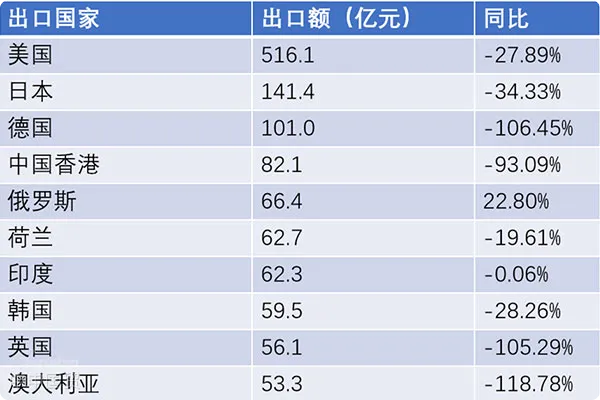
- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
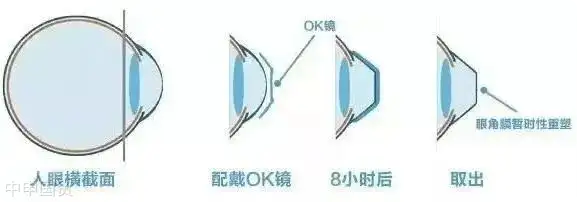
Rigid Gas Permeable (RGP) contact lenses, also known as OK lenses, are specially designed high - oxygen - permeable lenses. After wearing them for a period of time, the hydraulic pressure formed by the tear fluid between the lens and the cornea can change the shape of the cornea and temporarily reduce myopia.
RGP lenses are mainly made of silicone acrylate materials, which allow oxygen to pass through the lens to the cornea. This design makes RGP lenses more comfortable than traditional hard plastic contact lenses and also provides a clearer visual effect.
However, since orthokeratology lenses directly cover the corneal surface and are in close contact with eye tissues, there are certain risks in use. Therefore, this product is classified as Class IIIMedical Equipment, and there are strict requirements for its materials, production, and clinical applications. Before use, it needs to be evaluated for safety and effectiveness, and a medical device registration certificate must be obtained before production, sales, and use.
For Rigid Gas Permeable (RGP) contact lenses, the following are the applicable inspection standards, import document review requirements, and standardized declaration requirements:
I. Applicable Inspection Standards
RGP镜应符合GB 11417.2-2012 《眼科光学 接触镜 第2部分:硬性接触镜》和YY 0477-2016 《角膜塑形用硬性透气接触镜》等检验标准。主要检测项目包括:光学性能、几何尺寸要求、材料性能、生物相容性评价和微生物要求等。
II. Import Document Review Requirements
Orthokeratology lenses belong to Class III medical devices and should obtain the Medical Device Registration Certificate of the Peoples Republic of China or relevant supporting documents when imported. The customs will conduct consistency verification of the medical device registration certificate and review relevant supporting documents.
III. Standardized Declaration Requirements
When declaring orthokeratology lenses, refer to the commodity code: 90185000. The most - favored - nation import tariff rate is 0%, and the general tariff rate is 17%;Export DrawbackThe tax rate is 13%. Classification elements should include the product name, purpose, and principle. Price elements should include the brand (Chinese or foreign name), model, and medical device registration number (if any).
Related Recommendations
Category case
Contact Us
Email: service@sh-zhongshen.com
Related Recommendations
Contact via WeChat

© 2025. All Rights Reserved.沪ICP备2023007705号-2 PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912